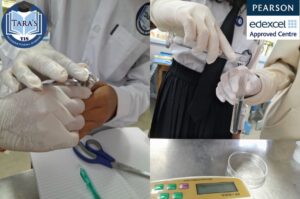
What is rusting?
The rusting of most of the iron in the environment is due to the chemical change caused by the reaction of oxygen and water in the air, resulting in corrosion (Hydarted Iron (lll) oxide).
The process of rust described now is taught to the students attending Key Stage 3 in TIS by experimenting with other reagents and teaching not only textual but also practical teaching of corrosion.
That’s why Tara’s International School is only teaching with a fully reliable method for children and women.
What is the process of rusting iron?
Rusting of iron is called corrosion and in the process of corrosion the iron gets converted into iron oxide and this is an example of chemical change.
Rusting is an oxidation reaction. The iron reacts with water and oxygen to form hydrated iron (III) oxide, which we see as rust. Iron and steel rust when they come into contact with water and oxygen – both are needed for rusting to occur.
What causes rusting?
Three things must be present for rusting to occur: iron, oxygen, and water. Rust forms when these three elements combine and create an electrochemical reaction.
[Iron + water + oxygen hydrated iron (III) oxide.